Abstract
The critical issue of human contamination in clean room environments, with a focus on compliance with Annex 1, Paragraph 7.2, of the European Union Guidelines to Good Manufacturing Practice (GMP). Personnel represent a significant contamination risk in sterile manufacturing, introducing both viable and non-viable particles into controlled environments. Annex 1 mandates stringent access control to mitigate these risks, including minimizing personnel in clean rooms and tracking entry and exit points.
NAV Team’s advanced people counting and access control solutions help manufacturers comply with these regulations. The system offers real-time monitoring, precise bi-directional people counting with 99.8% accuracy, and seamless integration with existing systems through a simple API. Additionally, prioritizes user privacy by using time-of-flight (TOF) technology, which avoids capturing images and ensures anonymous detection.
Highlights the importance of limiting human contamination and how NAV Team’s solutions reduce contamination risks while respecting privacy, offering a fully compliant system for clean room environments.
Introduction
Human contamination is one of the most significant challenges in maintaining clean room environments, particularly in pharmaceutical and sterile product manufacturing. According to Annex 1 of the European Union Guidelines to Good Manufacturing Practice (GMP) for Medicinal Products for Human and Veterinary Use, manufacturers are required to minimize contamination risks, particularly from personnel, as they are a primary source of contamination. Paragraph 7.2 specifically mandates strict control over personnel access in areas where contamination must be prevented. This white paper explores the key elements of controlled access and human contamination, focusing on compliance with Annex 1, Paragraph 7.2, and how NAV Team’s solutions can mitigate these risks through advanced technological integration.
The Risk of Human Contamination in Clean Rooms
Personnel present the highest contamination risk in clean rooms, introducing particles such as skin cells, hair, and microbes. The majority of particles in a clean room can be attributed to people, with research showing that human skin sheds approximately 500,000 particles per minute, and a single person can generate up to 10,000 particles per second during normal activities. This level of contamination, if left unchecked, can compromise product quality and safety, particularly in environments where sterile manufacturing is paramount.
In clean rooms, maintaining appropriate environmental conditions is crucial. Personnel movement, behavior, and even improper entry protocols can introduce non-viable (particles) and viable (microbes) contaminants. Scientific studies have identified personnel as the main source of contamination, responsible for up to 80% of airborne microbial contamination in clean rooms. As such, regulating human access is essential for maintaining compliance with Annex 1 standards.
Annex 1, Paragraph 7.2: Controlled Access Requirements
Annex 1 outlines specific expectations for personnel access to clean room environments, particularly in sterile areas. Paragraph 7.2 stresses the need for stringent entry protocols, requiring manufacturers to implement controlled access systems that reduce contamination risks. This includes:
- Minimizing personnel in clean rooms: Only essential personnel should be granted access.
- Control of entry and exit: Access points must be monitored, and personnel should undergo gowning procedures in airlocks to limit particle transfer.
- Tracking personnel movement: Records of personnel entry, exit, and activities should be maintained to ensure proper behavior and adherence to protocols.
The overarching goal of Paragraph 7.2 is to limit the introduction of contaminants by reducing human interaction with the controlled environment and ensuring strict adherence to operational protocols.
NAV Team’s Solutions for Controlled Access
NAV Team offers advanced solutions designed to help pharmaceutical and manufacturing companies comply with Annex 1, Paragraph 7.2, by integrating cutting-edge technologies for people counting, identification, and access control. These systems ensure that only authorized personnel can enter designated areas, and they provide real-time data on entry, exit, and zone occupancy. Our solution features include:
- Bi-directional People Counting: Our sensor technology allows for highly accurate monitoring of personnel movement in and out of clean rooms. With 99.8% accuracy, our solution ensures precise counting and tracking of individuals, preventing unauthorized access and overcrowding in controlled environments.
- Real-Time Monitoring and Alerts: NAV Team’s system offers real-time monitoring of personnel entering sterile areas, with alerts generated if maximum occupancy is exceeded or if access violations occur.
- Access Control Integration: Our system seamlessly integrates with existing access control systems, ensuring that personnel must follow strict protocols, such as gowning and handwashing, before entering controlled zones.
- API for Seamless Integration: NAV Team’s solutions are designed with a simple and secure API, making it easy to integrate with existing building management and access control systems. This flexibility allows manufacturers to incorporate our technology into their existing workflows without disruption, ensuring compliance with regulatory requirements while streamlining operations.
In addition to integrating with standard access control systems, NAV Team’s API also offers a simple interface to existing interlock systems. This ensures that entry into high- contamination-risk areas is tightly controlled and synchronized with interlock mechanisms, preventing unauthorized or simultaneous entry by multiple personnel. Such integrations are crucial in environments where contamination control and restricted access are vital. - Historical Data and Reporting: Data on personnel movements are stored and can be reviewed to track adherence to clean room protocols over time, offering manufacturers a clear audit trail for regulatory compliance.
- Anonymous Detection and Staf Differentiation: Our advanced radar-based technology can detect individuals anonymously while differentiating staff members based on role, ensuring compliance with restricted access policies while maintaining privacy.
Privacy: Protecting Personal Data
NAV Team utilizes a time-of-flight (TOF) sensor in its people counting solution, which is designed to prioritize user privacy without sacrificing accuracy. Unlike traditional surveillance systems, does not capture images or video footage of individuals. A state-of-the-art method that calculates the distance between objects by measuring the time it takes for light pulses to reflect off surfaces.
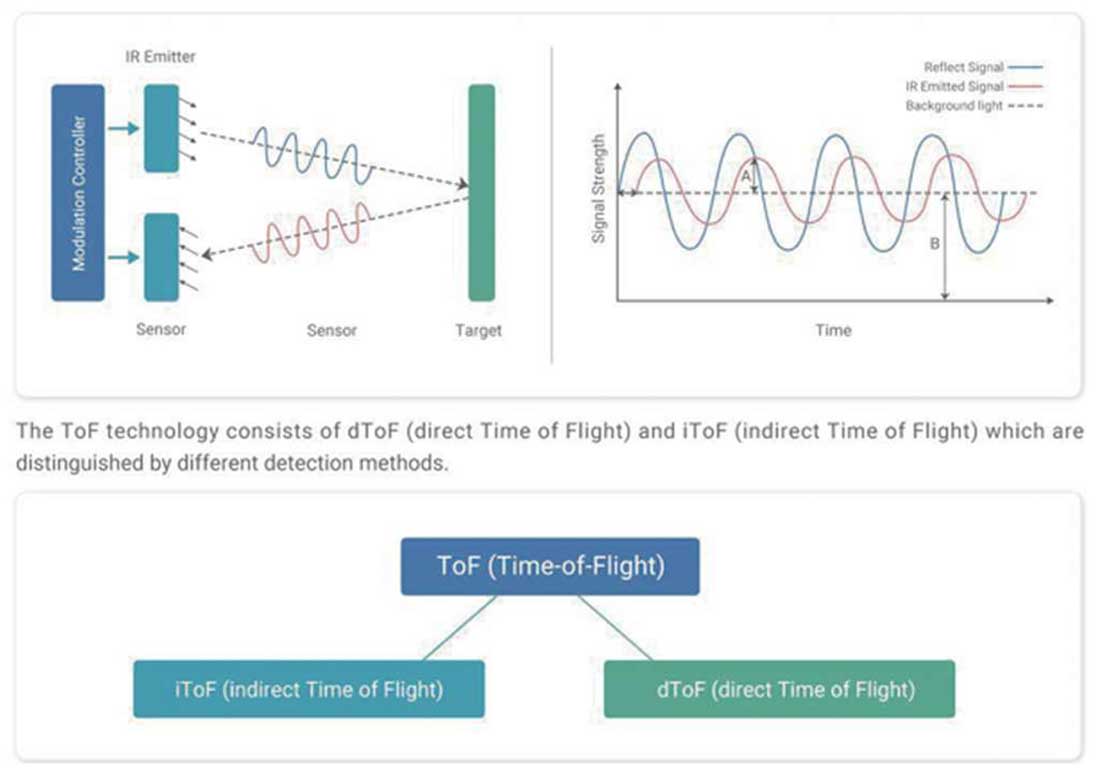
TOF technology ensures that only positional and movement data are recorded, without capturing any identifiable characteristics of individuals, such as facial features. This makes the solution ideal for environments where privacy is a concern, such as clean rooms where stringent data protection protocols must be followed. Additionally, TOF technology allows for highly accurate people counting, even in high-traffic environments, while ensuring compliance with GDPR and other privacy regulations.
Other key advantages of TOF technology is that it is not inhibited by lighting conditions, functioning accurately in both well-lit and dark environments. This ensures reliable performance under all conditions. Additionally, the TOF sensor offers up to 99.8% accuracy in people counting, even in high-traffic environments, providing manufacturers with confidence in the precision of their access control systems.
By leveraging TOF, NAV Team can guarantee accurate and anonymous detection, ensuring compliance with both clean room requirements and privacy standards. This feature provides peace of mind for manufacturers, knowing they are not only maintaining a sterile environment but also respecting the privacy of their personnel.
Real-Time Door Displays for Occupancy Management
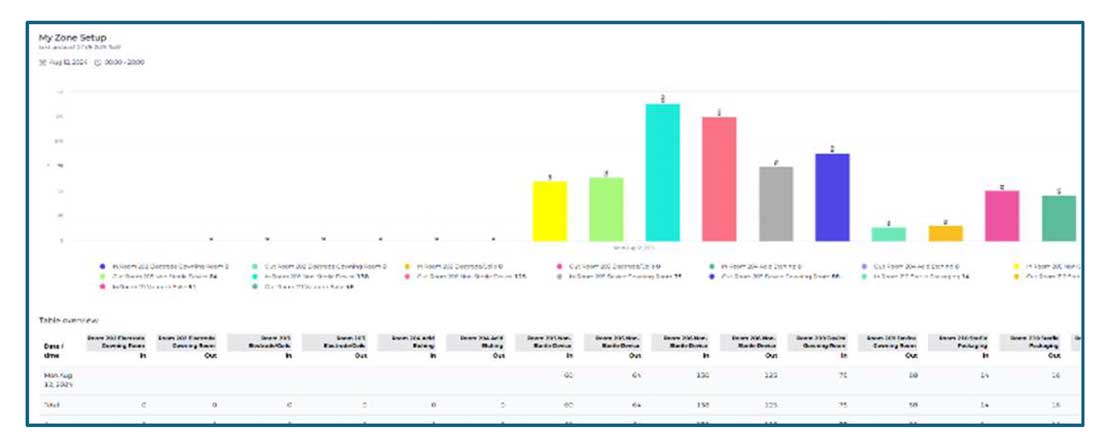
NAV Team’s system also includes real-time entrance door displays that provide a visual and immediate way to monitor current occupancy levels in controlled areas. These displays are strategically placed at entry points and are designed to enhance personnel awareness of clean room occupancy in real time.
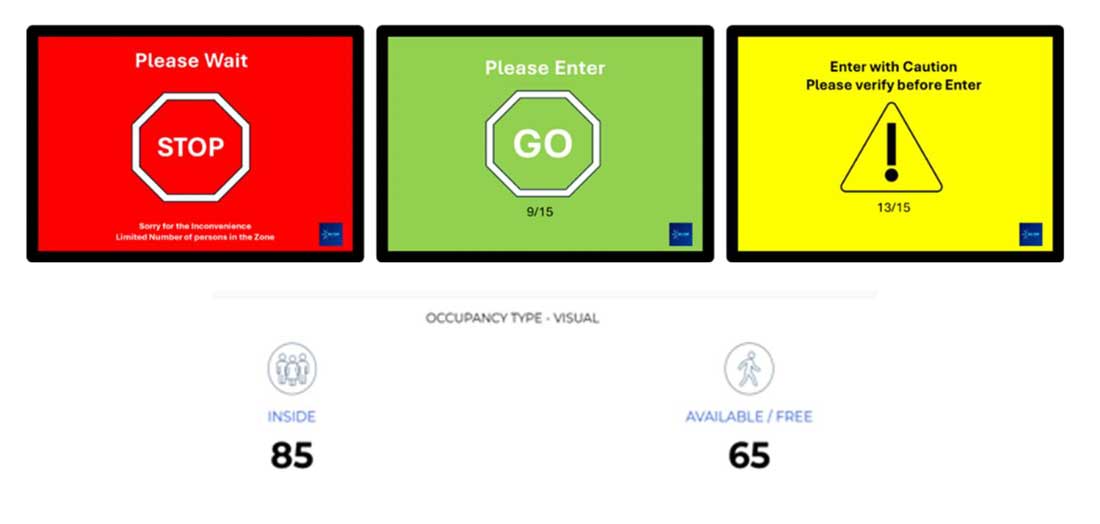
- Current Occupancy Display: The system shows the number of individuals currently in the room, allowing staff to remain informed of how many people are present without needing to manually check or track.
- Visual Caution Warnings: As the maximum occupancy limit is approached, the display shifts to a caution mode, providing a clear visual indicator to staff that they are nearing the capacity limit. This proactive warning system helps personnel manage access and avoid over-crowding.
- Max Occupancy Alerts: Once the maximum allowed occupancy is reached, the system will display a visual warning that entry is not permitted until someone exits. This feature is essential for ensuring compliance with both clean room requirements and occupancy safety standards. These real-time door displays not only improve adherence to clean room regulations but also facilitate smoother traffic control and access management, reducing the chances of contamination due to overcrowding.
Scientific Data Supporting Access Control
Research has shown that controlling human access to clean rooms significantly reduces the risk of contamination. A study by Whyte et al. demonstrated that well-controlled entry procedures, including proper gowning and controlled access systems, reduce microbial contamination by up to 90% in clean rooms. This underscores the importance of effective access control systems, such as those offered by NAV Team, in minimizing contamination risks.
Conclusion
In clean room environments, particularly in the pharmaceutical and sterile manufacturing industries, controlling human contamination is critical to maintaining product quality and safety. Annex 1, Paragraph 7.2, mandates strict access control to limit contamination, and NAV Team’s solutions are designed to help manufacturers meet these regulatory requirements. By leveraging our people counting, identification, and access control systems, which can seamlessly integrate with existing infrastructure through our API, companies can ensure compliance, reduce contamination risks, and protect the integrity of their sterile environments—all while safeguarding personal privacy through the use of TOF technology.
References
- Whyte, W. (2010). Cleanroom Technology: Fundamentals of Design, Testing, and Operation. John Wiley & Sons.
- Ljungqvist, B., & Reinmüller, B. (2012). Personnel Contamination in Clean Rooms. PDA Journal of Pharmaceutical Science and Technology.
- Whyte, W., et al. (2014). “Reduction of Microbial Contamination by Effective Gowning Procedures in Controlled Environments.” Journal of Contamination Control, 28(4), 216-223.
